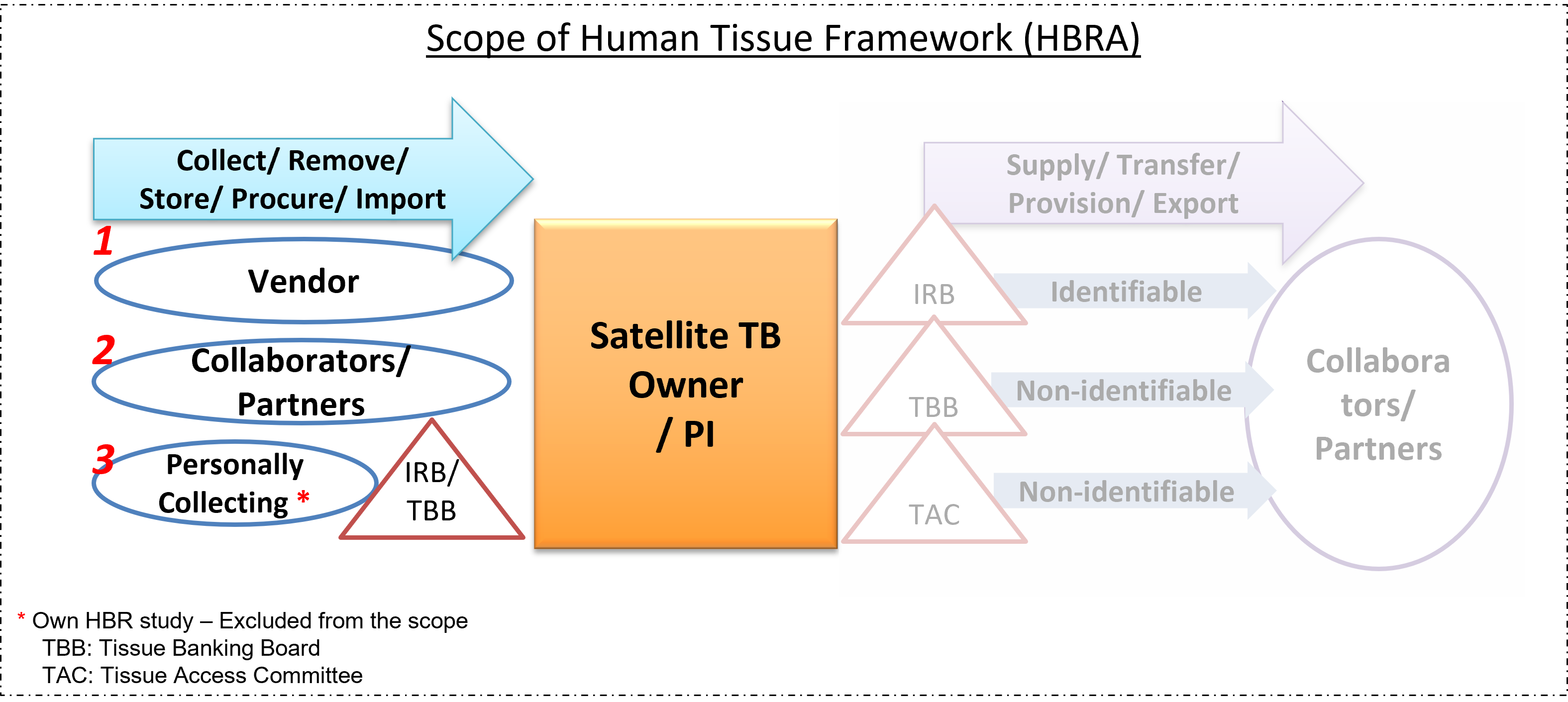
Collecting, Procuring, Importing, and Transfer-In of HBM
Effective 1 Jan 2017, HBRA prohibits commercial trading (i.e. buying and selling) of human tissue, as well as advertisements relating to such trading. This prohibition upholds the principle that human tissue should be obtained only through altruistic donations.
This may affect you if you make use of human tissue in the course of your work. If you have previously made arrangements to obtain and/or supply human tissue, please ensure that your arrangements are not in contravention of the prohibitions in the HBRA. Further information can be found on MOH's website. A guide is available here.
What about Human Blood Products?
Note that HBRA Section 32(6) and HOTA Section 14(4) have provisions for the purchase of human blood products and plasma fractions for research.
- For human blood, reimbursement for any cost and expenses incurred is not allowed under HOTA.
- Notwithstanding, blood products that have been subjected to processing or treatment are exempted from this prohibition under HOTA.
What about Tissue Products and Tissue Derivatives?
These are usually made from human biological material and would have already undergone substantial manipulation and processing. Where these are not considered to be human tissue, they can be bought and sold on a commercial basis. This would include cell lines.
While the HBRA prohibits commercial trading of human tissue, it allows for payment to reimburse the reasonable costs and expenses incurred in the process of collecting and supplying human tissue – including the removal of tissue from the donor, and the subsequent transportation, preparation, preservation, quality control and storage of the tissue. The payment must not be for the purchase or sale of the tissue itself.
In this regard, obtaining tissue from sources that are part of a tissue sharing network or exchange programme, whether local or international, and paying only reasonable costs and expenses in relation to such sourcing, would generally be permitted. In contrast, sourcing for tissue from foreign commercial suppliers or tissue banks that routinely charge a price for selling the tissue itself, should be done cautiously.
When making arrangements to obtain human tissue, it is advisable to clarify the arrangements with the supplier providing the human tissue to ensure that the supply of the tissue is on a cost-recovery basis. These arrangements and transactions with the supplier should be documented accordingly (e.g. written in the invoice or material transfer agreement).
Please also see section below on the "Transfer-in of HT from Collaborators / Partners" for the other documentary evidence required.
IMPORTANT NOTE
- Before obtaining individually identifiable human tissues from commercial sources, please ensure that NTU-IRB approval has been obtained.
- Before obtaining anonymised human tissues from commercial sources, please ensure that you have registered the tissue banking activities in NTU's Ethics Review Management Portal (ERMP).
Local Partners: For the transfer-in of human tissues from local collaborators/ partners, PIs are to obtain documentary evidence from the source that consent was obtained in accordance with HBRA requirements.
Overseas Partners: For the transfer-in of human tissues from overseas collaborators/ partners, PIs are to obtain documentary evidence from the source that consent was obtained in accordance with the legal/ ethical requirements of the country where the tissue came from.
Documentary Evidence:
Overseas partners/collaborators/suppliers: This documentation must be obtained prior to the import of the human tissues. It could be in the form of email correspondences or official documents (e.g. Material Transfer Agreements, Research Collaboration Agreements) stating that consent has been obtained for each sample in accordance with the legal or ethical requirements of the country from where the human tissues came from. The email correspondences or official documents should clearly specify the detail of each samples including (but not limited to) the sample code, type, and quantity covered by the document.
The airway bill, delivery order/tax invoice) should also include the Tissue/Donor code and the tissue type, and be archived for records keeping by the PI.
Local partners/collaborators/suppliers: This documentation must be obtained prior to the transfer-in of the human tissues and must either be in the form of email correspondences or an official document (e.g. Material Transfer Agreements, Research Collaboration Agreements) stating that consent has been obtained for each sample in accordance with HBRA requirements. The email correspondences or official documents should clearly specify the details of each sample including (but not limited to) the sample code, type, and quantity covered by the document.
Click here to download the template form to fulfil the documentary evidence requirement.
IMPORTANT NOTE
- Before obtaining individually identifiable human tissues from local collaborators/partners, please ensure that NTU-IRB approval has been obtained.
- Before obtaining anonymised human tissues from local collaborators/partners please ensure that you have registered the tissue banking activities in NTU's Ethics Review Management Portal (ERMP).
For PIs who are self-collecting human tissues for your research (i.e. recruitment of participants/donors), appropriate consent must be obtained from your tissue donors. The consent form used must contain all the Section 12(2) elements under HBRA. This must be approved by NTU-IRB before tissue collection can proceed.
Although PIs are not required to register as Tissue Banking sites in NTU for their own Human Biomedical Research(HBR) projects, PIs should note that the storage of leftover human tissues for future research is considered a Tissue Banking activity under the HBRA. Accordingly, when closing the HBR study, PIs who intend to retain such human tissues for future use must register their tissue banking activities through NTU’s Ethics Review Management Portal (ERMP). This ensures compliance with all applicable legislative and institutional requirements.
Safety and Welfare of Donors
- Tissue collection should be conducted by only qualified and trained personnel (e.g. certified phlebotomist).
- Measures must be put in place to control and prevent the spread of communicable diseases.
- Put in place quality control procedures and maintenance of equipment
