Exporting and Supplying of HBM
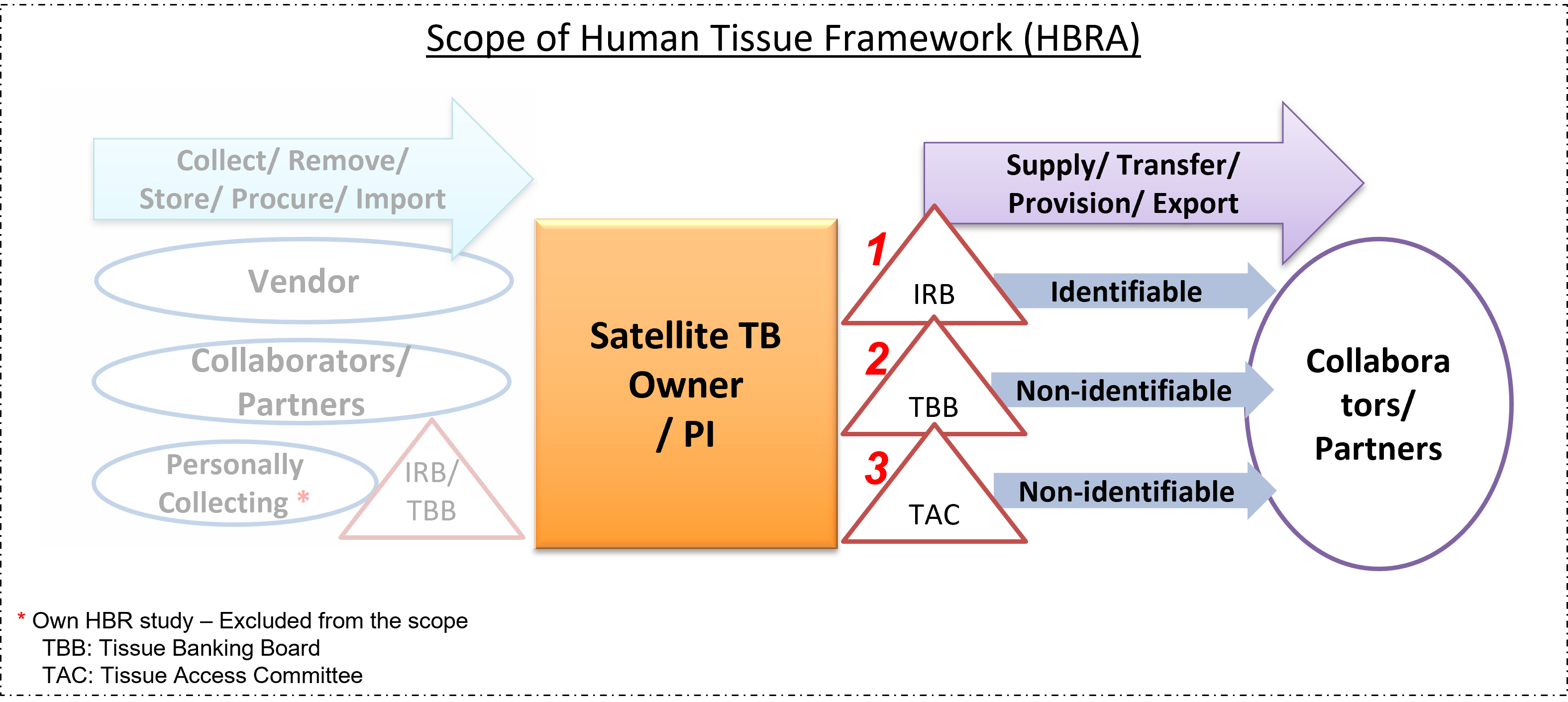
Transfer and Supplying of Human Biological Materials (HBM)
[#1 above] Before any tissue bank or PI can supply individually-identifiable tissue for use in research, the PI must ensure that: (1) an IRB has approved the proposed research that
the tissue would be used for; and (2) documentary evidence* is provided by the receiving party that the receiving party will ensure that the intended use of the tissue is in accordance
with any restrictions/conditions of the donors' appropriate consent.
[#2 and #3 above] If non-identifiable tissue is to be supplied for use in research, the PI must ensure that: (1) an IRB has approved the proposed research that
the tissue would be used for; or NTU-TBB (or TAC if available) has approved the proposed research based on scientific merit; and (2) documentary evidence* is
provided by the receiving party that the receiving party will ensure that the intended use of the tissue is in accordance with any restrictions/conditions of the donors' appropriate consent.
*Note: PIs are to use the latest Material Transfer Agreement (MTA) template provided by LSO to fulfil the "documentary evidence" requirement under HBRA. The MTA must be signed by the recipient, in addition to the institutional signatory.
Tissue Release Form
To facilitate the release of tissues by any tissue banker or PI, a Tissue Release Form can be used by Tissue Requestors. Once endorsed by the tissue banker or PI, the form will be reviewed by NTU-TBB via ServiceNow (Research Services > Tissue Request), in accordance with HTF (HBRA) requirements. Tissue Bankers or PIs are to retain a copy of the completed signed form from your tissue recipient (or receiving party).
Export of Human Biological Materials (HBM)
In addition to the above:
If individually-identifiable tissue is to be exported from Singapore, explicit consent must be obtained from the donor for the export of his/her
HBM.
De-identified tissue may be exported even if consent from the tissue donor had not been obtained for its export. However, if the donor has stipulated that he/she did not wish for his/her tissue to be exported, his/her wishes should be respected.
