Nano Forest for Efficient Water Splitting
Web of Science's Hot Paper by Prof Hongjin Fan and Prof Cheng Yang

Achievement
Recently, Prof Cheng Yang from Tsinghua University, Shenzhen International Graduate School and Prof Hongjin Fan from Nanyang Technological University jointly made an important progress and published their work in the prestigious journal Energy & Environmental Science. They tackled this challenge by developing a nickel-iron (NiFe) alloy nanowire array by employing a magnetic field assisted synthesis route. The obtained alloy nanowires are vertically aligned resembling a nano forest. Although nickel or iron material has been the common catalyst in industry, such NiFe alloy nanowire arrays produced by the magnetic field regulation method has obvious uniqueness. The fine geometry of the hierarchical electrode can substantially boost the charge and mass (reactants and oxygen bubbles) transfer. They can provide channels for efficient transport of ions, electrons, and gas bubble during the high-current OER process. In addition, a thin nickel-iron hydroxide shell layer (1-5 nm) in situ formed on the surface via a nano-confinement effect shows superior intrinsic catalytic property which is supplemented by the NiFe alloy core during service. With this unique design, the catalyst exhibits outstanding performance of OER and outperform all the previously reported catalysts, especially during operation at large current densities. Specifically, the “nano forest” catalyst can stably work for more than 120 h with only about 0.25 and 0.26 V overpotentials at the current density of 500 and 1000 mA cm-2, respectively.
In order to construct a full electrolyzer for complete water splitting (i.e., including both HER and OER), the researchers also fabricated pure nickel nanowires array cathode via the same magnetic field assisted method. The full-water electrolytic cell can reach a current of 1000 mA cm-2 under only 1.76 V, which is lower than state-of-the-art industrial catalysts (more than 1.8 V). This work provides a new and promising approach to develop highly active and stable electrolytic water catalysts towards industrial hydrogen production from water.
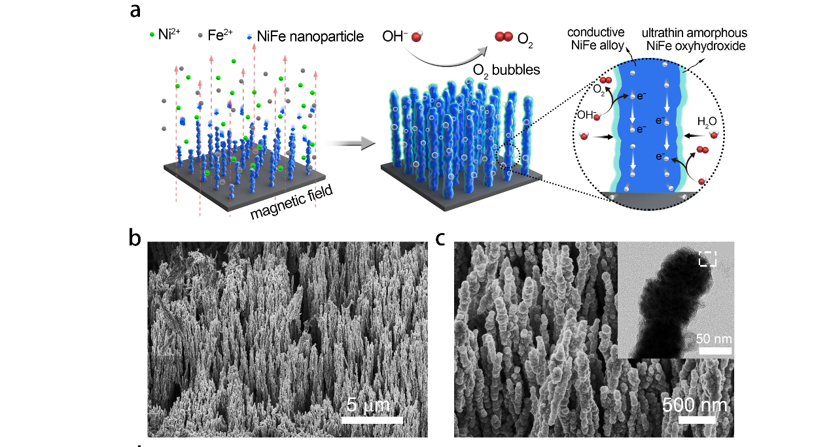
(a) Schematic of the growth of NiFe alloy nanowires by self-assembly of nanoparticles driven by external magnetic field, and the catalytic function of the nanowires array for oxygen evolution reaction.
(b-c) SEM images of the fabricated NiFe alloy nanowires array. The inset TEM image shows a thin amorphous shell which provides catalytic activity.
References
Caiwu Liang, Peichao Zou, Adeela Nairan, Yongqi Zhang, Jiaxing Liu, Kangwei Liu, Shengyu Hu, Feiyu Kang, Hong Jin Fan and Cheng Yang, “Exceptional Performance of Hierarchical Ni-Fe Oxyhydroxide@NiFe Alloy Nanowire Array Electrocatalysts for Large Current Density Water Splitting”, Energy & Environmental Science, 2020, 13(1), 86-95, DOI 10.1039/c9ee02388g.
Web of Science’s Hot Paper | 224 Citations (as of 8 December 2021)





.tmb-listing.jpg?Culture=en&sfvrsn=c45c3c7e_1)