Research Feature of the Month
Each month, we spotlight a standout publication by our faculty that exemplifies the innovation and impact of MSE research. From advancing sustainable materials to breakthrough applications in healthcare and energy, these works reflect our commitment to pushing the frontiers of materials science.
Sept 2025
Spin Regulation Boosts Ammonia Oxidation Efficiency for a Cleaner Energy Future
This feature was prepared in collaboration with NTU MSE's Prof. Jason Xu and Research Fellow, Dr. Wu Qian.
Ammonia isn’t just a fertiliser — it could be tomorrow’s green fuel.
Hydrogen is one of the world’s cleanest fuels, offering an alternative to coal, oil, and gas. Ammonia has attracted growing attention as a promising hydrogen carrier, since it can be liquefied and stored in bulk under relatively mild conditions. The challenge lies in efficiently decomposing ammonia to release hydrogen. This process normally requires a lot of energy and doesn’t run smoothly. Imagine trying to play a team sport when everyone is running in different directions — that’s how the atoms behave in this reaction. Finding a way to get them “in sync” will make the process faster, less wasteful, and truly viable for clean energy.
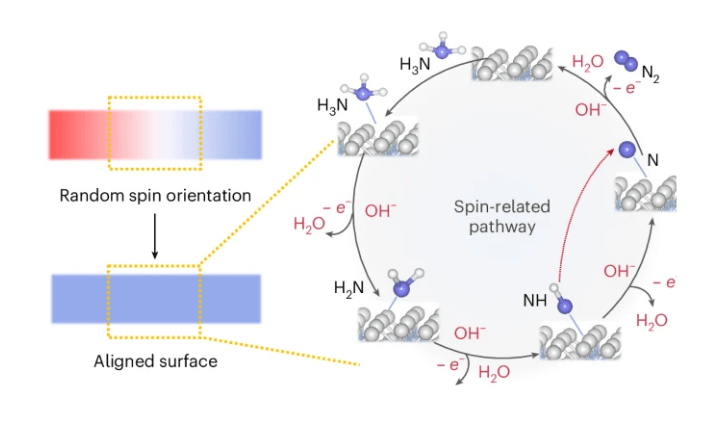
NTU MSE researchers have shown that cooperative spin alignment can make the electrochemical ammonia oxidation reaction (AOR) more efficient — a critical step in unlocking hydrogen as a clean fuel. Using specially engineered cobalt–platinum thin-film catalysts, the team demonstrated that when nitrogen intermediates (N and NH) align their spins with the magnetic substrate, the energy barrier for their dimerisation drops dramatically, establishing a spin-regulated kinetic route to promote electrochemical ammonia decomposition.
This work introduces spin dynamics as a new design principle for electrocatalysis. Instead of relying solely on catalyst composition or structure, researchers can now consider how magnetic ordering at the atomic level influences reaction kinetics. The study not only clarifies a long-debated step in the ammonia oxidation mechanism but also establishes a precedent for integrating quantum spin effects into catalyst engineering — offering collaborators and peers with a foundation for developing next-generation catalysts for hydrogen conversion and sustainable chemical synthesis.
Building on this mechanism, the research opens pathways to:
Extend spin alignment strategies to other electrochemical reactions where intermediate coupling is a bottleneck.
Develop catalysts with tailored magnetic domains to enhance stability and reactivity for cleaner energy conversion.
Inspire interdisciplinary approaches that merge materials science, magnetism, and quantum chemistry to tackle challenges in sustainable fuels.