Radio-Afterglow Nanoprobes (RANPs) for Early Diagnosis and Precision Treatment of Cancer
Synopsis
This next-generation theranostic platform uses Organic Radio-Afterglow Nanoprobes (RANPs) for deep-tissue cancer imaging and therapy. By converting X-ray energy into long-lasting optical afterglow and reactive oxygen species, RANPs enable early tumour detection and precise radiodynamic therapy at low radiation doses. The tumour-activatable nanoprobe (tRANP) allows accurate identification and surgical removal of micro-tumours as small as 1 mm³. Offering a safer, more effective alternative to conventional radiotherapy, this technology holds strong potential for personalised cancer treatment. The team seeks clinical and industry partners in oncology, nanomedicine translation, and radiotherapy development.
Opportunity
Radiation therapy is a cornerstone of clinical cancer treatment. However, high doses often result in unavoidable damage to healthy tissues, as well as tumour resistance and metastasis, which limit its wider clinical application. Clinical imaging techniques have limitations in detecting very small tumours (< 5 mm) located deep within tissues, hindering early diagnosis and timely medical intervention.
This technology introduces a next-generation theranostic platform for cancer imaging and therapy based on Organic Radio-Afterglow Nanoprobes (RANPs), designed for ultrasensitive deep-tissue cancer imaging with long afterglow signals and biomarker activation, enabling early detection and surgical precision/treatment. RANPs integrate three functional components:
- Radioabsorber – converts X-ray energy into radioluminescence.
- Radiosensitiser – activated by radioluminescence to generate singlet oxygen (¹O₂).
- Radioafterglow substrate – reacts with ¹O₂ to form intermediates that emit persistent afterglow.
This platform also introduces a tumour-specific biomarker-activatable nanoprobe (tRANP) that allows for highly specific tumour detection and potential surgical removal of minute tumours (as small as 1 mm³) at an X-ray dose comparable to a single CT scan. The highly reactive oxygen species (¹O₂) generated from low-dose X-ray irradiation enable Radiodynamic Therapy (RDT), which can eradicate tumours and reduce metastasis, demonstrating its potential for cancer treatment. This technology presents a strong fit to support personalised medicine.
The technology owner is seeking to collaborate with:
- Clinical and medical partners specialising in early detection, surgical and radiation oncology.
- Partners with experience in translating nanomedicine or theranostic agents into clinical trials.
- Companies working on nanoparticle radiosensitisers, imaging agents, or radiotherapy platforms.
Technology
- Cascade X-ray energy transfer by organic molecules: By fine-tuning radioluminescence and ¹O₂ transfer among key nanoparticle components, this technology has, for the first time, demonstrated the mechanism for the efficient conversion of X-ray energy into optical afterglow signals and ¹O₂ generation, enabling tumour theranostics in murine models.
- Modular and customisable design: The modular composition and well-defined mechanism of RANPs allow for the loading of various optical agents, enabling tunable emission wavelengths, radioafterglow brightness, and ROS generation efficiency to meet different cancer theranostic needs.
- Ultrasensitive cancer detection at an incipient stage: Imaging and surgical removal of minute tumours (below 1 mm³) can be achieved at an X-ray dose comparable to a clinical CT scan (mGy level) and 20 times lower than that required for inorganic materials.
- Precision cancer therapy with minimal X-ray dosages: The efficient radiodynamic ¹O₂ generation by tRANPs enables complete tumour eradication at X-ray doses lower than those used in clinical radiotherapy and with drug doses one to two orders of magnitude lower than those required for most inorganic agents, thereby prolonging survival while minimising radiation-related side effects.
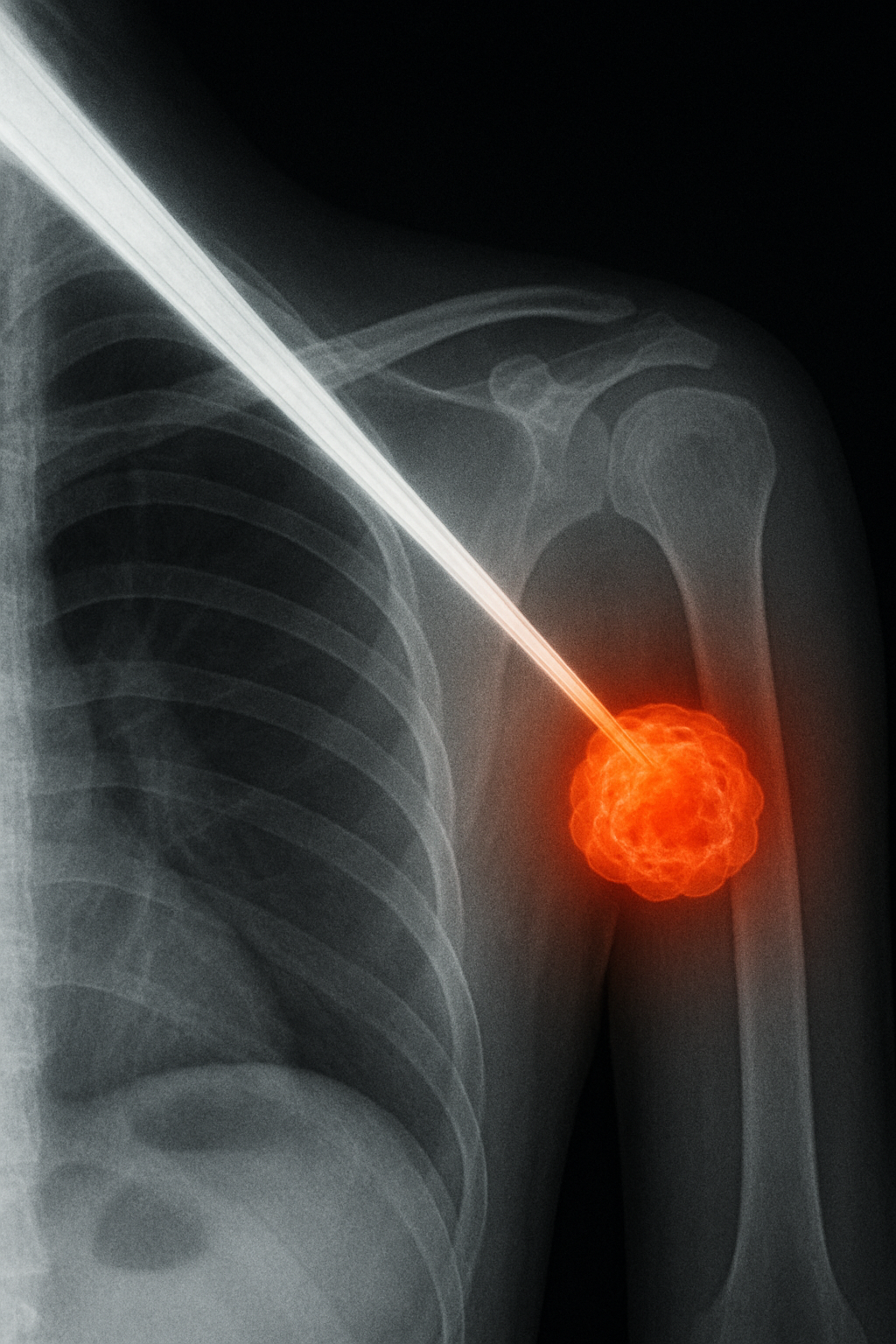
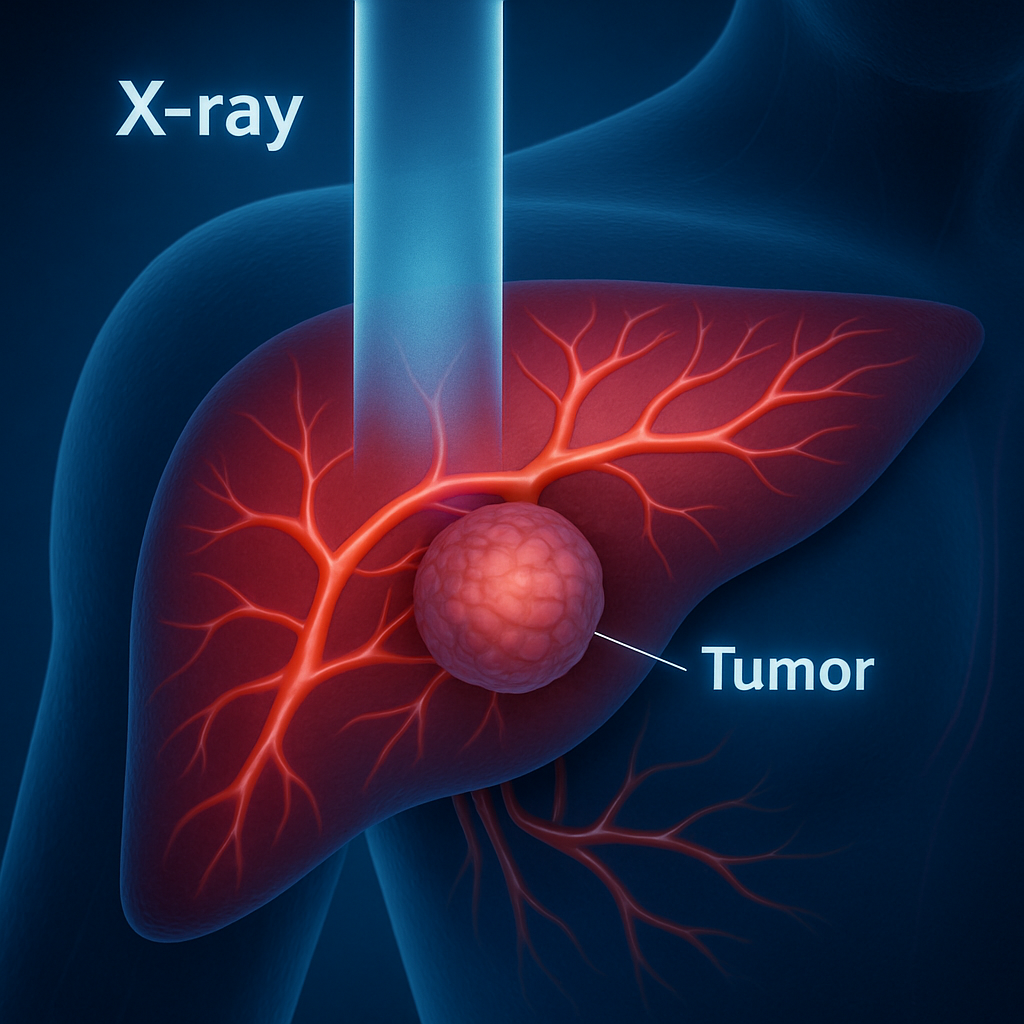
Applications & Advantages
Applications
- Oncology Diagnostics: Addresses the unmet need for early-stage tumour detection, particularly in deep-seated tissues where existing imaging techniques (MRI, CT, PET) have limitations. RANPs can be tailored to respond to specific disease biomarkers, facilitating the detection of other cancers and diseases (e.g. infectious diseases, diabetes).
- Tumour Staging: RANP signals correlate with increasing biomarker levels and tumour size.
- Cancer Therapeutics: Provides a safer, more effective alternative or complement to radiation therapy by reducing dosage and enhancing treatment outcomes. Due to their high X-ray sensitivity, RANPs can generate reactive oxygen species (ROS) for Radiodynamic Therapy (RDT) of cancer and potentially infectious diseases at any tissue depth reachable by X-rays, delivering precise and safe treatment of deep-seated diseases at a minimal dosage.
- Surgical Applications: Enables real-time intraoperative guidance for precise tumour removal and reduces recurrence rates.
- Bimodal Imaging Probes: The similarity in X-ray parameters between computed tomography scanners and RANPs allows for the simultaneous acquisition of both anatomical and molecular information on diseases in a single computed tomography scan. Combined with whole-body CT scanning, low-dose X-rays can activate RANPs accumulated in potential metastatic sites, assisting in metastasis screening.
Advantages
- Dual-Functionality Theranostics: This study developed an integrated organic nanoplatform for simultaneous cancer diagnosis and therapy. By adjusting the radiation dose from mGy to Gy levels, it enables rapid switching between imaging and treatment, achieving both imaging and therapy after a single injection of a single probe.
- Minimized Side Effects: In contrast to traditional radiotherapy, which typically requires prolonged treatment cycles, high radiation doses, and causes notable side effects, RANP-mediated tumour therapy operates at radiation doses ~10 times lower than conventional radiotherapy, reducing collateral damage to healthy tissues and demonstrating substantial clinical potential, especially for X-ray-sensitive organs (e.g., liver).
- Tumour-Specific Targeting: RANPs offer exceptional sensitivity for tumour detection, capable of identifying tumours as small as 1 mm³— a level of precision that many current clinical imaging modalities cannot reach. When combined with standard hospital CT scanners, this technology can significantly enhance diagnostic sensitivity and accuracy, holding great promise for early cancer screening.
- Persistent Signal Advantage: Long signal half-lives improve imaging resolution, reduce background noise compared to real-time fluorescence imaging, and enable functionality at tissue depths of up to 15 cm.
- Biocompatible: Compared to inorganic materials, which often pose challenges in metabolism and potential toxicity, the components of the organic nanoparticles in this system exhibit excellent biocompatibility and safety, and they can be efficiently metabolised and cleared from the body.

-and-dr-dorrain-low-from-ntu-singapore's-lkcmedicine.tmb-listing.jpg?Culture=en&sfvrsn=b389b500_1)
.tmb-listing.jpg?Culture=en&sfvrsn=82921582_1)

.tmb-listing.jpg?Culture=en&sfvrsn=ba129532_1)