Seeing at the smallest scale
Using a novel cryo-EM technique, NTU scientists determine the structures of protein-DNA complexes that are important for DNA organisation and key cellular processes.
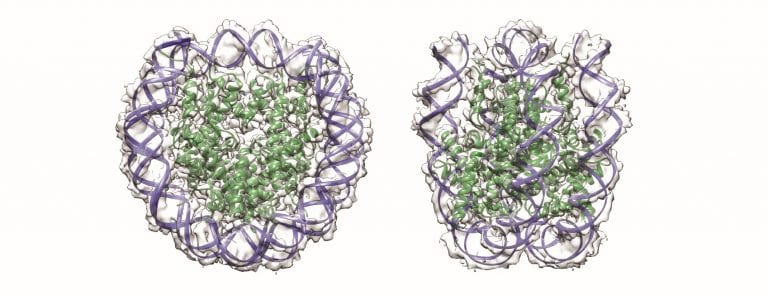
Illustration of nucleosome core particles. Credit: Sara Sandin.
Using cryo-electron microscopy (cryo-EM), a technique that won its inventors the 2017 Nobel Prize in Chemistry, researchers at the NTU Institute of Structural Biology have determined protein structures at unprecedented resolution.
In a study published in Nucleic Acids Research as a breakthrough article, Asst Prof Sara Sandin and her colleagues determined the structure of nucleosome core particles—small protein-DNA complexes that are important for DNA organisation and key cellular processes such as gene expression.
“Using the novel Volta phase plate cryo-EM technique provided significant contrast enhancement that hugely increased detection of molecules in ice,” says Asst Prof Sandin. “The 3.9 Å resolution allowed us to clearly identify individual particles and even substructures such as amino acid side chains, as well as the conformations of free DNA molecules.”
The team’s breakthrough could make it possible to determine DNA-binding protein complexes and other challenging intracellular biological structures at near-atomic resolution, the researchers say. Two brand new state-of-the-art scanning and transmission electron microscopes equipped with Volta phase plates and direct electron detectors are currently being installed at NTU for that purpose.














/enri-thumbnails/careeropportunities1f0caf1c-a12d-479c-be7c-3c04e085c617.tmb-mega-menu.jpg?Culture=en&sfvrsn=d7261e3b_1)

/cradle-thumbnails/research-capabilities1516d0ba63aa44f0b4ee77a8c05263b2.tmb-mega-menu.jpg?Culture=en&sfvrsn=1bc94f8_1)

7e6fdc03-9018-4d08-9a98-8a21acbc37ba.tmb-mega-menu.jpg?Culture=en&sfvrsn=7deaf618_1)






