Energetic Polymers
Energetic Binders
Polymer based binders are integral parts of energetic material composites. Binder functions as the fuel as well as provides structural integrity to the propellant charge. The polymer, Hydroxy terminated polybutadiene (HTPB) is the workhorse of propellant industries, with excellent low temperature mechanical properties, and wide operating temperatures. However, since HTPB is an inert component, it requires very high oxidizer content (>80%) to achieve acceptable performance level of the propellant charge. Higher oxidizer content directly translated to a higher sensitivity to external stimuli of the propellant charge and also incurred additional processing costs. Hence, research efforts have been directed at introducing energetic groups such as azido (-N3) or nitrato (-ONO2) to the polymer binder with the aim of achieving better performance at low solid contents.
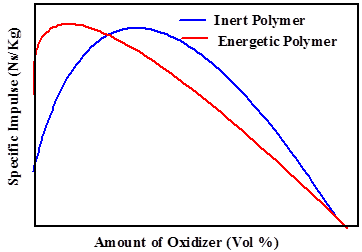
These polymers containing energetic groups are known as energetic polymers. Examples of energetic polymers containing azido groups are Glycidyl azide polymer (GAP) and Poly (Bis-azido methyl oxetane) (PBAMO). Poly (Nitrato methyl methyl oxetane) (PNIMMO) and Poly (Glycidyl Nitrate) (PGLYN) are examples of nitrato polymers. Both class of polymers release considerable amount of energy, under the influence of external stimuli. These polymers differ among themselves in the mechanism of energy release. Nitrato polymers produce energy by oxidation reactions while azido polymers generate energy by the high heat of formation of the azido groups.
Energetic Thermoplastic Elastomers
With increasing complexities in the munition designs, commensurate with the performance requirements, structure and processing demands placed on energetic material composites have become more and more vigorous.
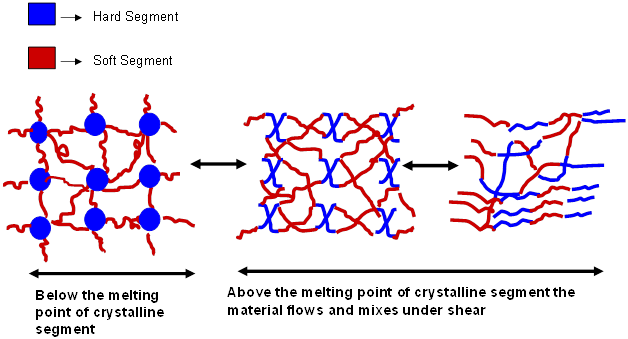
Research efforts have been directed at copolymerizing crystalline hard energetic polymer segments along with the soft energetic polymer backbone to improve the mechanical and performance properties. The crystalline hard segments are made up of Poly Bis Azido Methyl Oxetane (BAMO) units. Such materials result in energetic thermoplastic elastomers (ETPE’s). At room temperature the hard segments form physical crosslinks with the amorphous segments. The crystalline component functions as a thermally stable segment which melts and flows under shear. Thus ETPE’s could be melt cast, allows good processing flexibility and completely eliminates the use of chemical crosslinking agents.
 EnRI has successfully developed synthetic routes for the preparation of energetic thermoplastic elastomer; GAP-PBAMO copolymers with varying molecular weights as binder for melt casting applications.
EnRI has successfully developed synthetic routes for the preparation of energetic thermoplastic elastomer; GAP-PBAMO copolymers with varying molecular weights as binder for melt casting applications.
Energetic Plasticizers
The role of plasticizers in energetic material composites are (a) to modify mechanical characteristics of composites by improving the elongation at break; (b) to improve on the low temperature properties of the composites . Plasticizers are classified into non- energetic and energetic. Examples of non-energetic plasticizers include the esters, acetyl triethyl citrate, diethyl adipate, diethyl sebacate and diethyloctoate. Energetic plasticizers are nitro compounds and nitrate esters. Examples include trimethyloethane trinitrate (TMETN) and trimethylene glycol dinitrate (TEGDN) or bisdinitropropyl formal and acetal mixture (BDNPF/A).
One of the recurring problems with all these plasticizers is their migration to the surface of the formulation, thus negating the plasticization effect. Recent promising approach is to use low molecular weight energetic polymers as the plasticizer. EnRI has successfully synthesized energetic polymer plasticizers namely; low molecular weight GAP and azido terminated GAP (GAP-A).

These energetic polymer based plasticizers are structurally similar to binder matrix and resist the migration due to enhanced miscibility. Low molecular weight (Mw= 650 to 800 g/mole) GAP polymers have been developed as efficient plasticizers to improve the mechanical properties of GAP binder. However, the plasticization effect will be hampered, if the terminal hydroxyl groups of GAP plasticizer react with the isocyanate curing agent. Azido terminated GAP plasticizers (GAPA) are also developed, in which the terminal hydroxyl groups of the GAP plasticizer are converted to azido groups.
