Drug Delivery Systems For Glaucoma And Cornea
Collaborator: Singapore Eye Research Institute (SERI)
The overall goal of this project is to develop sustained release platforms for the delivery of ocular drugs to the anterior and posterior segments of the eye. Most drugs have poor ocular penetration due to the multiple physiological barriers of the eye and the retention of drugs is also difficult to achieve due to rapid clearance. Hence, the development of biodegradable subconjunctival implants to control drug release could circumvent these two major problems. Our group has successfully developed implants for the delivery of a number of corticosteroids and beta-blockers used to address ocular pathologies (including uveitis and glaucoma) and to address postoperative inflammation. These biodegradable microfilms exhibit sustained drug release and have shown to be safe and well tolerated in animal models. All these formulations are in the pre-clinical stages of development and could provide an alternative therapy for eye diseases in the future.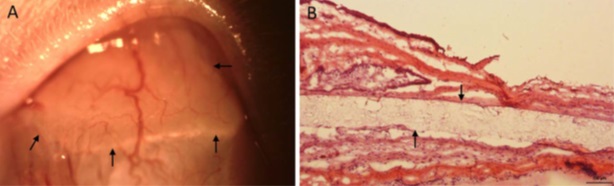 Figure 1: Clinical and histological evaluation of prednisolone acetate microfilm implantation. (A) Slit lamp photo showing the subconjunctivally-implanted microfilm at 4 weeks. Arrows indicate the implanted microfilm. (B) Histological section with H&E staining at 4 weeks. Arrows indicate the implanted microfilm
Figure 1: Clinical and histological evaluation of prednisolone acetate microfilm implantation. (A) Slit lamp photo showing the subconjunctivally-implanted microfilm at 4 weeks. Arrows indicate the implanted microfilm. (B) Histological section with H&E staining at 4 weeks. Arrows indicate the implanted microfilm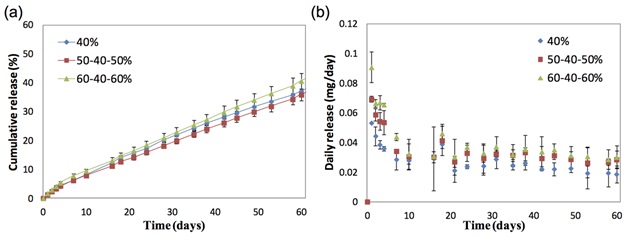 Figure 2: In vitro and In vivo release profiles depicting (a) cumulative release of prednisolone acetate (%) and (b) daily release of prednisolone acetate (mg/day) amount from three formulations of poly(d-, l-lactide-co--caprolactone) films loaded with prednisolone acetate.
Figure 2: In vitro and In vivo release profiles depicting (a) cumulative release of prednisolone acetate (%) and (b) daily release of prednisolone acetate (mg/day) amount from three formulations of poly(d-, l-lactide-co--caprolactone) films loaded with prednisolone acetate.
Funding agency: National Medical Research Council
Publications:
(1) Ang M, Ng XW, Wong C, Yan P, Chee S-P, et al. Evaluation of a prednisolone acetate-loaded subconjunctival implant for the treatment of recurrent uveitis in a rabbit model. PLoS ONE, 9 (5), e97555. doi:10.1371/journal.pone.0097555 (2014).
(2) Liu Y-C, Peng Y, Lwin NC, Venkatraman SS, Wong TT, et al. A biodegradable, sustained-released, prednisolone acetate microfilm drug delivery system effectively prolongs corneal allograft survival in the rat keratoplasty model. PLoS ONE, 8 (8), e70419. doi:10.1371/journal.pone.0070419 (2013).
(3) Peng Y., Ang M., Foo S., Lee W.S., Ma Z., Venkatraman S. S., Wong T. T. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS One, 6 (7), e22507. doi: 10.1371/journal.pone.0022507 (2011).














/enri-thumbnails/careeropportunities1f0caf1c-a12d-479c-be7c-3c04e085c617.tmb-mega-menu.jpg?Culture=en&sfvrsn=d7261e3b_1)

/cradle-thumbnails/research-capabilities1516d0ba63aa44f0b4ee77a8c05263b2.tmb-mega-menu.jpg?Culture=en&sfvrsn=1bc94f8_1)

7e6fdc03-9018-4d08-9a98-8a21acbc37ba.tmb-mega-menu.jpg?Culture=en&sfvrsn=7deaf618_1)
