Development and Application of Sustained Drug Release Technology for the Delivery of Nucleic Acid Molecules for the Prevention of Post-Operative Scarring in Glaucoma
Collaborator: Dr. Tina Wong, Singapore Eye Research Institute (SERI)
Glaucoma is a progressive optic neuropathy which affects approximately 70 million people world-wide, of whom 6.7 million suffer from bilateral blindness; there is a high demand for an effective treatment. Currently, the elevated intraocular pressure (IOP) occurring in glaucoma, which can result in blindness, is reduced by glaucoma filtration surgery.
Success is limited due to post-operative wound healing response, which involves the development of subconjunctival fibrosis, scarring over the sclerostomy with subsequent bleb failure and raised IOP.
The persistent post-operative inflammatory response continues to be a major frustration to patients and the ophthalmologists entrusted with their care.
The elevated secreted protein, acidic and rich in cysteine (SPARC) protein expression is associated with tissue scarring, fibrosis and is a target for therapeutic approach, but efficient and safe delivery of siRNA remains a challenge. Here we investigate the feasibility of encapsulating SPARC-siRNA in the bilayers of layer-by-layer nanoparticles (NPs) with poly(L-arginine) and dextran as polyelectrolytes.
Cellular binding and uptake of LbL NPs as well as siRNA delivery were studied in mouse and human fibroblasts. Up to 95% siGLO-siRNA and SPARC-siRNA were efficiently coated onto hydroxyapatite NPs with 200nm in diameter. These NPs are efficiently internalized into fibroblasts and release the siRNA for up to two weeks and significantly reduced the SPARC expression levels without any toxic effects. One of the optimised formulations is administered in mouse glaucoma surgical model and observed efficient silencing of the SPARC and collagen levels. Such non-viral vectors based on multilayered particles are safe and efficient in high therapeutic loading of various siRNA to target specific gene as a new treatment modality for a range of human diseases.
| Zeta potential changes during LbL assembly |
|---|
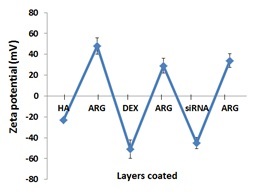 |
| Relative SPARC-mRNA levels indicating efficient silencing |
|---|
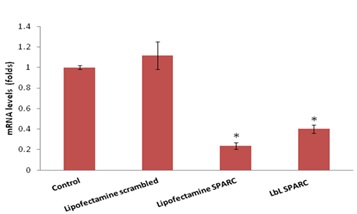 |
| Multilayered nanoparticles as non-viral vectors for siRNA delivery |
|---|
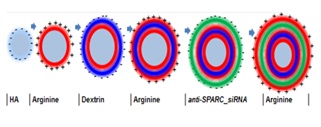 |
| siRNA-loaded nanoparticles |
|---|
 |
| Uptake and intracellular release of siRNA in fibroblasts |
|---|
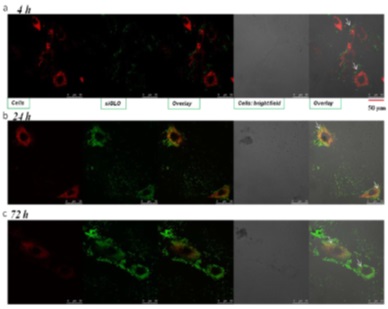 |
Funding agency: National Medical Research Council
Publication:
Yang Fei Tan, R.C. Mundargi, Min Hui Averil Chen, Jacqueline Lessig, Björn Neu, Subbu S. Venkatraman, Tina T. Wong. Layer-by-layer nanoparticles as an efficient siRNA delivery vehicle for SPARC silencing, Small, 10, 1790–1798 (2014).














/enri-thumbnails/careeropportunities1f0caf1c-a12d-479c-be7c-3c04e085c617.tmb-mega-menu.jpg?Culture=en&sfvrsn=d7261e3b_1)

/cradle-thumbnails/research-capabilities1516d0ba63aa44f0b4ee77a8c05263b2.tmb-mega-menu.jpg?Culture=en&sfvrsn=1bc94f8_1)

7e6fdc03-9018-4d08-9a98-8a21acbc37ba.tmb-mega-menu.jpg?Culture=en&sfvrsn=7deaf618_1)
